Mass spectroscopy is a spectroscopy technique used to determine the molecular mass of the compounds. The mass spectroscopy is used for both qualitative and quantitative study of chemical substances. It is most accurate method amongst all other spectroscopic technique.
- The mass spectroscopy technique helped to identify of isotopes of substances.
- It is an efficient technique to explain the chemical composition of a sample or molecule.
- It can calculate the purity of substances.
PRINCIPLE: based on Newton’s second law of motion and momentum , a mass spectroscopy uses this property of matter to plot ions of varying masses on a mass spectrum. Mass is relevant to the inertia and acceleration of body. This principle is applied to the aspect where ions with different mass to charge ration (m/z) are deflected by different angles in an electric and magnetic field. The molecules are converted into gaseous form and a beam of electron will be bombarded which leads to removal of 1electron from the molecule. Due to removal of electron, the molecule become ionized. These are separated on the basis of mass to charge ratio.
M + e– = M+ + 2e–
All ions are accelerated across the same distance by same force, therefore they have same kinetic energy (KE) determined by acceleration voltage of the instrument (V) and charge of the ion (z).
KE= Zv= ½ mv2
As charged ions move through magnetic field, they bend in an arc to follow circular path of certain radium which is directly proportional to applied magnetic field. The magnetic force (fh) on the ions are balanced by centripetal force and is given as-
Fh= zvH=mv2/r
Rearranging above equations
v= Hzr/m
we get-
KE= zV= mv2/2
m/z= H2r2/2V
or radius of arc
r=1/H √(2mv/z)
the ions of certain m/z value have same path radius which can be determined if both magnetic and electric field magnitude H, acceleration voltage V are held constant.
Thus, the principle of mass spectroscopy is base on mass to charge ratio.
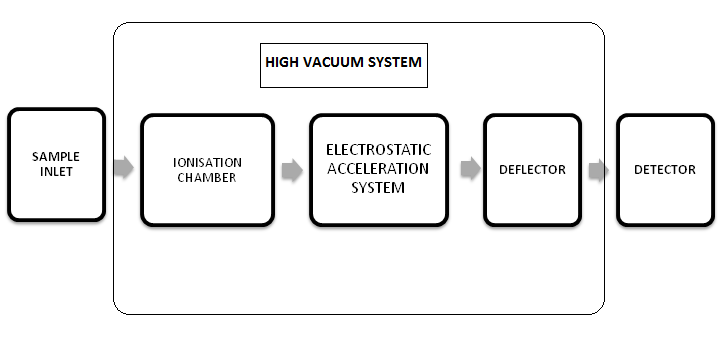
INSTRUMENTATION OF MASS SPECTROSCOPY :
- Sample inlet: as a mass spectrometer should have vapour sample and to ensure the sample enters the ionization chamber at a constant rate, the sample is converted into gaseous state in the inlet system. The volatile liquids and solids may be vaporized directly within the ionization chamber. The heating coils are there near sample inlet which produce temperature so that the liquid and solid samples get converted into gaseous form under suitable atmospheric pressure.
The rate at which sample is introduced into an ionization chamber must remain constant so that relative abundances of different species in mass spectrum can be determined. A sample of 1u mole is required.
To handle different types of sample, different inlet system are employed
2. Ion source/ ionization chamber: From inlet system, the sample is introduced into the ionization chamber where a beam of electron is put across the molecules of sample the molecules being ionized. The collision between molecules and electrons result in production of well defined fragments. Each fragment carries a definite charge and moves through the instrument as a sample charged molecule. The minimum potential required to convert molecules into ions is known as ionization potential , value of ionization potential is minimum 8 to 15eV.modernforensic.in
Ionization technique for solid
- Field desorption
- Plasma desorption
- Fast atom bombardment
- Secondary ion
Ionization technique for liquid
- Matrix assisted laser desorption (MALDI)
- Electro spray
- Atmospheric pressure chemical ionization
Ionization technique for gas
- Electron ionization
- Photo ionization
- Chemical ionization

3. Electrostatic acceleration system: the positive ions formed in the ionization chamber are withdrawn by electric field. Resolving the ions which are formed in the ionization source according to their mass-to-charge (m/z) ratio. The electrode potential of ion acceleration system is 8-10KV. The velocity selector selects the electrons that have specific velocity.
4. Deflector: the accelerated electron from the electric field enters the magnetic field, the force of magnetic field requires them to move in a curved path. The radius of the curvature r is dependent upon the mass of the ion, the accelerating voltage, the electron charge of the ion, and the strength of the magnetic field. The mass to charge ratio and radius of curvature is interdependent. All particles of greater or lesser m/z ratio will strike the side of separation tube and will be neutralized. The magnetic field and electric field provide in the electrostatic acceleration system are perpendicular to each other.
5. Detector: the beam of electron striking the detector are detected on the basis of mass to charge (m/z) ratio. Common detectors used in mass spectrometers are the photomultiplier tube, faraday cup, electron multiplier tube etc. based on the mass spectrum produced by charged ions, we can identify the atoms or molecules consisting the sample by comparing them with known masses or through a characteristic fragmentation pattern.
6. High vacuum system: mass spectroscopy uses gas phase ions for analysis, they operate in high vacuum typically 10-2 to 10-5 Pa, maintained by pumping system. The ionization chamber, deflector and electrostatic acceleration system are maintained under high vacuum so that the ions travels smoothly to the detector or the also to minimize the changes of collision of ions with other molecules so that they do not react, scatter or fragment.

APPLLICATIONS:
- Toxicological analysis: in cases involving poisons or drugs. Mass spectroscopy determine if the toxic substance is present in samples of subject’s tissue or body and if so in what concentration.
- Trace evidence: trace evidences like fibers, glass fragments, paint sample may be found in the crime scene. Mass spectroscopy can determine the composition of various dyes used in the fibers, set of polymer used in making of paint. This information can lead investigation to a particular manufacturer, who may be able to narrow down the investigation process.
- Arson investigation: if there is any particle or exotic, volatile compound found on the crime scene. Mass spectroscopy can lead break down any residue and provide an accurate report of the molecules present.
- Explosive residue: another area where mass spectroscopy plays an important role. The chemical residue, small fragments, physical explosives may be present in the crime scene. The mass spectroscopy can narrow the investigation by identify the particular molecular makeup of the explosives. The commercial explosives utilizes unique mix of chemical the analysis may be used to identify the type of materials used.
For more updates, subscribe to our blog.






