The word chromatography is based on greek word chromo meaning “ color “ and graphos meaning “writing”. The chromatography is technique of separation of individual component of the mixture by an equilibrium distribution between two phases.
It is a primary separation tool, resolving multi component mixtures of minor, major and trace components into its individual fractions. it is a non- destructive in nature and may be applied both qualitatively and quantitatively.
It was first invented by a botanist M. Tswett in the year 1906 in Warsaw. He successfully separates xanthophyll, chlorophyll, and various other colored compound through a column of calcium carbonate by percolating the extract of plant. He termed the bands of colors as chromatogram.
The technique involves two basic phases; mobile phase- the liquid or gas that moves in a chromatographic system carrying different system into it. Stationary phase- the phase over the mixture flows or over which mobile phase passes.
The chromatographic technique consists of following steps:
- Retention or absorption of substance on the stationary phase
- Separation of the absorbed substances by the mobile phase.
- Elution, the separated substance recovered from the chromatographic chamber.
- Qualitative and quantitative analysis of eluted substances.
Principle of chromatography
This is the technique in which the sample is combined with the mobile phase and pumped through the stationary phase. Depending upon the polarity, molecular interactions, they spend more time with the stationary phase and thus retarded as lesser or greater extent. Different components have different polarity will travel at different rates because the solvent moves, causing their separation.
The position of the migrated spots is indicated by Rf value:
Rf = distance travelled by the solute from the start line / distance travelled by the solvent from the start line
R is the function of the partition coefficient. It is constant for a given substance, provided the conditions are kept constant with respect to temperature, type of paper, Duration and direction of development.
The Rf defines the movement of the sample relative to the solvent in a given chromatographic system.
Rf value of a system depends upon the following factors:
The solvent used
The medium used for separation
The nature of the mixture
The temperature and
The size of the vessel in which operation is carried out.
It is possible to compare the value of different substances by keeping the above factor constant.
TYPES OF CHROMATOGRAPHY
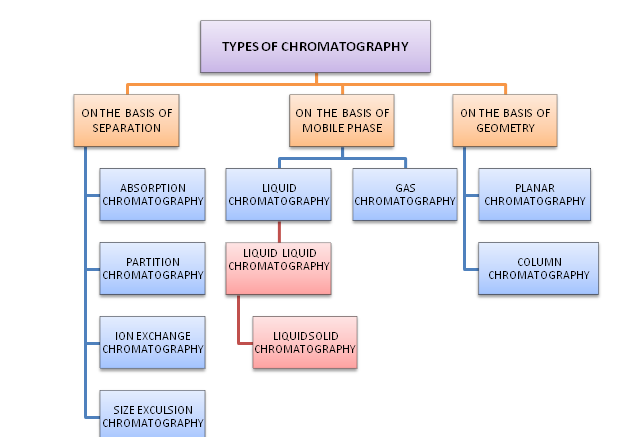
Types of chromatography on the basis of separation
- Adsorption chromatography: It is based on adsorption phenomenon (surface phenomenon). The stationary phase is solid and mobile phase may be liquid or gas. The gas or liquid adsorbs on the stationary phase (solid surface). It is the oldest method of chromatography technique. The mixture of liquid gets separated when passes through the adsorbent bed that adsorbs the sample at different rates.
ADSORBENT: the substance which is porous and high surface area to provide to adsorb the sample to its surface by intermolecular force. Some commonly used adsorbent are silica gel, alumina, modified silica gel H etc.
2. Partition chromatography: there will be the partition between the liquid- liquid / liquid or gas. The stationary phase in partition chromatography is always polar or non volatile liquid for better stability of stationary phase. The mobile phase may be liquid or gas. The partition chromatography technique was introduced by Richard Laurence Millington Synge and Archer Martin In the year 1940. It is also known as liquid – liquid chromatography (LLC) or gas-liquid chromatography (GLC).
The partition between the stationary phase and mobile phase and on the basis of partition coefficient the separation takes place. If the sample is non polar as compared to stationary phase, it will elute out first and if the sample is polar, it will elute out later.
3. Ion exchange chromatography: ion exchange chromatography allows separation of polar molecule and ions is based on their affinity of ion exchanger. It is the reversible There Is a ion exchange resin that where fixed ions are bond with covalent bond.
Stationary phase is cellulose or dextrone matrix on which some fixed ions are attached by covalent bond and mobile phase is generally low to medium conductivity solution. The adsorption of molecules to the solid support is attached by ionic interaction between fixed ions and the sample molecules. Molecules have strong interaction elute later than compared to the molecules have weaker interaction elute out first.
4. Size exclusion chromatography: the separation takes place on the basis of the molecules of different sizes. It is generally used to separate the biological compounds and to determine molecular weight of the compounds. When the sample mixed with liquid sample is applied to the chromatographic column which contains microscopic solid samples known as beds. This column bed acts as a sieve or tracer. Larger molecule does not penetrate or partially penetrate the pores and are excluded out first as compared to the smaller molecules.
Types of chromatography in the basis of mobile phase
1. Liquid chromatography
- Liquid liquid chromatography
- Liquid solid chromatography
2. Gas chromatography
a. Liquid liquid chromatography: this technique is based on partition chromatography. The stationary phase and mobile phase both are in liquid phase. In this paper chromatography will be there in which paper is used as a stationary phase. The paper is made up of alpha cellulose which contains 6% of moisture and thus this moisture act as a stationary phase.

b. Liquid solid chromatography: most of the chromatography techniques fall under this type. A technique which separates solute based on their adsorption to a support. The liquid is the mobile phase and stationary phase is solid.
TLC (thin layer chromatography): TLC is defined as a way of separation or identification of a mixture of components into individual components by using adsorbent solid spread over a plate and a liquid as a mobile phase. This technique was first introduced by Izmailov and Shraiber in 1938.
HPTLC, Ion exchange chromatography, HPTLC, size exclusion chromatography etc.
2. Gas chromatography: Gas chromatography is an analytical technique that separates the chemical components (organic molecules or gases) of a sample mixture and detect them to determine their presence or absence and how much is present. The mobile phase (moving phase) is a carrier gas, usually an inert gas such as helium, nitrogen and stationary phase is a microscopic layer of a liquid a polymer on an inert solid support, called column.
Type of chromatography on the basis of geometry
Planar chromatography: in this type of chromatography, stationary phase is arranged in the form of planar and mobile phase moves by capillary action. Thin layer and paper chromatography are the most widely used planar chromatography.
Column chromatography: The stationary phase is held in a tube through which the mobile phase is forced under pressure or by gravity. The adsorbent packed in the glass column and a solvent or mobile phase moves slowly through the packed column. The stationary phase is the adsorbent substance over the column and mobile phase is liquid. the components of the mixture with lower affinity to stationary phase travel fast as compared to the components with higher affinity with the stationary phase.
subscribe to our blog





